In the study published in Journal Nature on September 26, entitled “Structural basis of archaeal FttA-dependent transcription termination”, Prof. WANG Chengyuan’s group at the Shanghai Institute of Immunity and Infection of the Chinese Academy of Sciences, together with Prof. Richard Ebright’s group at Rutgers University and Prof. Thomas J. Santangelo’s group at Colorado State University of USA, reported the structures of FttA-dependent transcription termination complexes from Thermococcus kodakarensis. The structures revealed the molecular mechanism of FttA mediated RNA polymerase transcription termination in archaea.
The ribonuclease FttA (also known as aCPSF and aCPSF1) is responsible for transcription termination at majority of transcription units in archaea in vivo. FttA is a homologue of INTS11 and CPSF73 in eukaryote. The researchers reported the cryo-EM structure of a Thermococcus kodakarensis transcription pre-termination complex comprising FttA, Spt4, Spt5, and a transcription elongation complex (TEC). The structure showed that FttA interacted with the TEC in a manner that enabled RNA to proceed directly from the TEC RNA-exit channel to the FttA catalytic center. FttA then performed the 5'®3' exonucleolytic cleavage of RNA, thus applying mechanical force to the TEC and triggering termination.

Figure 1. Structure of the FttA pre-termination complex (Tko FttA(H255A/H591A)–Spt4–Spt5–TEC52). (Image by Chengyuan WANG)
Specifically, the structures revealed functional analogy between bacterial and archaeal factor-dependent termination, functional homology between archaeal and eukaryotic factor-dependent termination, and fundamental mechanistic similarities in factor-dependent termination in the three domains of life: bacterial, archaeal, and eukaryotic.
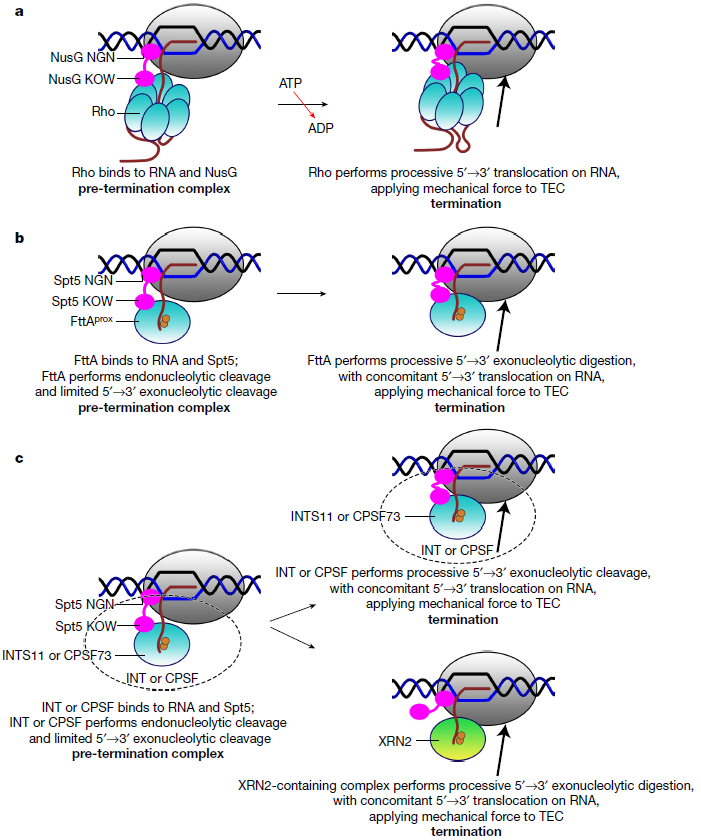
Figure 2. Mechanisms of bacterial, archaeal, and eukaryotic factor-dependent termination. (Image by Chengyuan WANG)
The structure further revealed that Spt5 bridges FttA and the TEC, explaining how Spt5 stimulates FttA-dependent termination which may also mediate transcription-translation coupling in archaea. The structures provided a foundation for further structural and functional understanding of archaeal transcription termination and archaeal transcription-translation-coupling quality control.

