On 13 August, Dr. Yanjie Chao, Principal Investigator at the Shanghai Institute of Immunity and Infection of the Chinese Academy of Sciences, published a research paper entitled "RNA interactome of hypervirulent Klebsiella pneumoniae reveals a small RNA inhibitor of capsular mucoviscosity and virulence" in the journal Nature Communications. Focusing on the pathogenic mechanism of hypervirulent and drug-resistant superbugs, the study revealed a small noncoding RNA regulatory network of hypervirulent Klebsiella pneumoniae using the recently developed RNA-RNA interactome profiling approach (iRIL-seq), and discovered that small RNAs effectively inhibited superbug infection by targeting a novel virulence mechanism.
Hypervirulent Klebsiella pneumoniae (HvKP) causes severe infections in healthy humans, leading to diseases such as pneumonia, liver abscess, meningitis, sepsis, etc. The emergence of HvKP has been reported in at least 16 different countries around the world, and has recently been listed by the World Health Organisation (WHO) as a priority pathogen that poses risk for a future pandemic. The major virulence factor of HvKP is the highly viscous polysaccharide on the bacterial surface, which helps the bacterium escape the host immune system. Understanding how the synthesis of capsule polysaccharides is regulated and how the capsular viscosity is generated are key to understand the pathogenesis of HvKP.
Small noncoding RNAs (sRNAs) are the most abundant class of post-transcriptional regulators in bacterial pathogens, which regulate the expression of target mRNAs via direct RNA-RNA interactions. In this study, the authors established a global RNA-RNA interaction network in hypervirulent Klebsiella pneumoniae using iRIL-seq, a recently developed RNA-RNA interactome profiling technology (Nature Commun, 2023,14:8106), and discovered that sRNAs controls the synthesis of capsule polysaccharides and virulence.
Based on the RNA-RNA interactome data, the researchers screened more than 20 sRNAs and found that ArcZ had the strongest inhibitory effect on capsular mucoviscosity. ArcZ also significantly inhibited the pathogenicity of hypervirulent Klebsiella pneumoniae in mice. The inhibitory effect of ArcZ was highly conserved, independent of the genotype and capsule type of strains. ArcZ significantly inhibited the capsular mucoviscosity in several different hypervirulent and drug-resistant clinical strains, suggesting that ArcZ is a potent RNA inhibitor of superbugs with therapeutic potential in clinics.
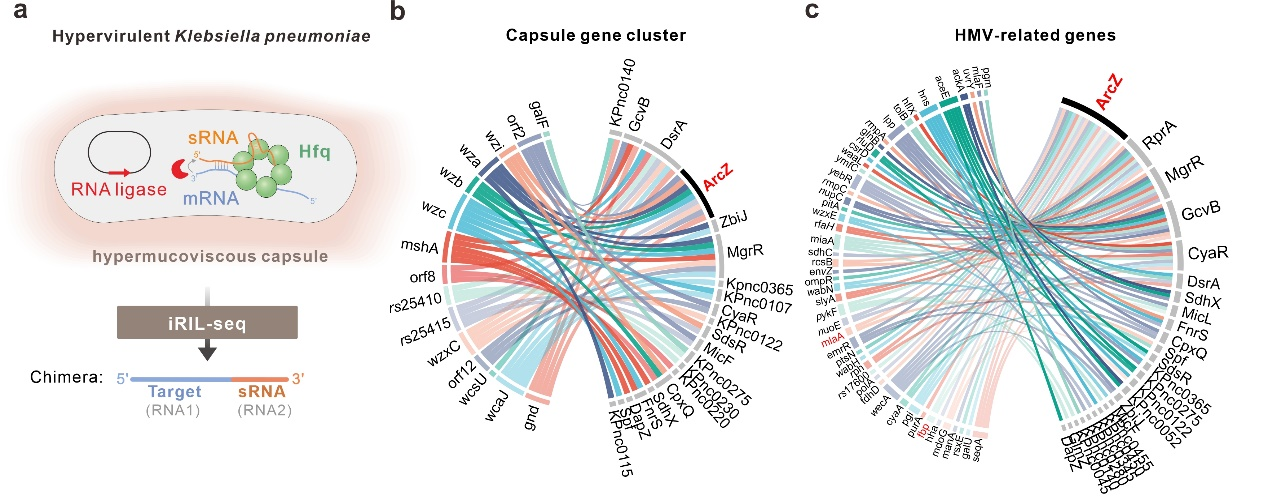
Figure 1. RNA-RNA interactome profiling reveals the RNA regulatory network of capsule polysaccharide genes in hypervirulent Klebsiella pneumoniae. (Image by SIII)
The study further identified the expression of ArcZ is activated by carbon metabolism signals and the related transcription factor CRP; and identified the ArcZ downstream targets genes are mlaA and fbp, which encode the outer membrane phospholipid transporter protein MlaA and the fructose-1,6-bisphosphatase Fbp, respectively. Researchers demonstrated that ArcZ inhibits the expression of mlaA and fbp mRNAs by a direct RNA-RNA basepairing interaction mechanism, revealing a novel CRP-ArcZ-MlaA capsular hypermucoviscosity regulatory pathway.
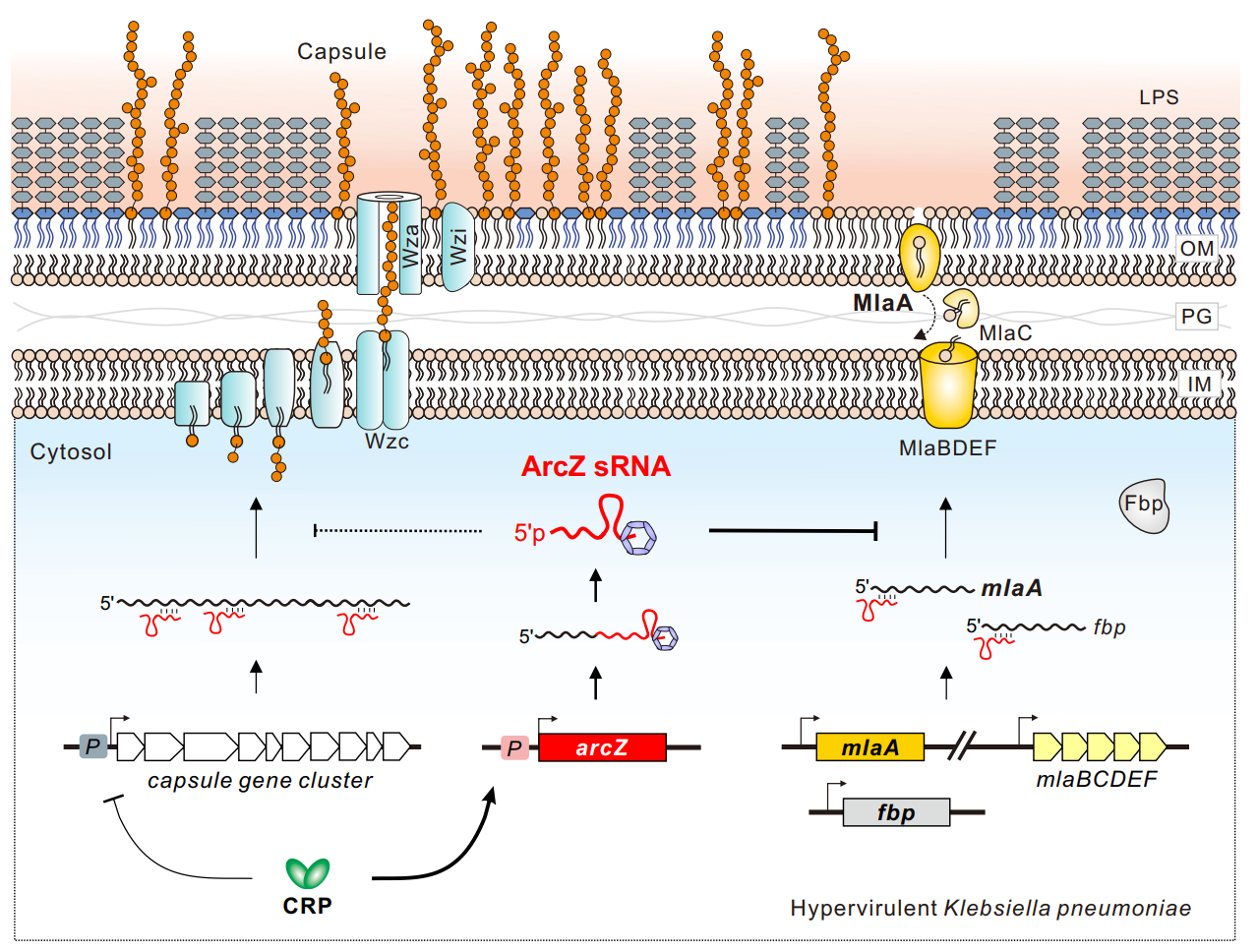
Figure 2. Molecular mechanism how small noncoding RNA ArcZ represses capsular mucoviscosity in hypervirulent Klebsiella pneumoniae. (Image by SIII)
Since the outer membrane phospholipid transporter protein MlaA is not directly involved in the synthesis of capsule polysaccharides, the researchers further elucidated the molecular mechanism by which MlaA regulates capsular mucoviscosity. By gene knockout, single-copy gene complementation and mutation of key amino acid residuals, the study found that the MlaA protein and its phospholipid transporter activity is essential for both capsular mucoviscosity and pathogenicity, establishing that MlaA is a novel virulence factor in hypervirulent Klebsiella pneumoniae. Inactivation of MlaA not only led to a significant reduction in the capsular mucoviscosity, but also a significant reduction in bacterial virulence in mice, revealing a virulence attenuation approach potentially useful for vaccine development.
In summary, this study not only revealed a novel virulence mechanism underlying the hypervirulent superbug infections, but also identified a small noncoding RNA molecule that neutralized this virulence mechanism. These discoveries will be extremely helpful for the development of novel anti-infective drugs and effective vaccines to curb superbug infections in the future.
Contact:
Dr. Yanjie Chao
Shanghai Institute of Immunity and Infection,
Chinese Academy of Sciences
Email: yjchao@ips.ac.cn
Reference: https://doi.org/10.1038/s41467-024-51213-z
Link of CAS: https://english.cas.cn/newsroom/research_news/life/202408/t20240822_683734.shtml

