In the study published in the journal Nature Communications on February 6, 2023, entitled Microbiota-derived acetate enhances host antiviral response via NLRP3, Prof. MENG Guangxun’s group at the Shanghai Institute of Immunity and Infection of the Chinese Academy of Sciences reported their new research.
Pathogenic viral infections represent a major challenge to human health. Host immune responses to respiratory viruses are closely associated with microbiome and metabolism via the gut-lung axis. It has been known that host defense against influenza A virus (IAV) involves activation of the NLRP3 inflammasome, however, mechanisms behind the protective function of NLRP3 are not fully known.
Here Prof. MENG’s group shows that an isolated bacterial strain, Bifidobacterium pseudolongum NjM1, enriched in the gut microbiota of Nlrp3?/? mice, protects wild-type but not Nlrp3 deficient mice against IAV infection. This effect depends on the enhanced production of type I interferon (IFN-I) mediated by NjM1-derived acetate.
Application of exogenous acetate reproduces the protective effect of NjM1. Mechanistically, NLRP3 bridges GPR43 and MAVS, and promotes the oligomerization and signalling of MAVS; while acetate enhances MAVS aggregation upon GPR43 engagement, leading to elevated IFN-I production.
Thus, their data support a model of NLRP3 mediating enhanced induction of IFN-I via acetate-producing bacterium and suggest that the acetate-GPR43-NLRP3-MAVS-IFN-I signalling axis is a potential therapeutic target against respiratory viral infections.
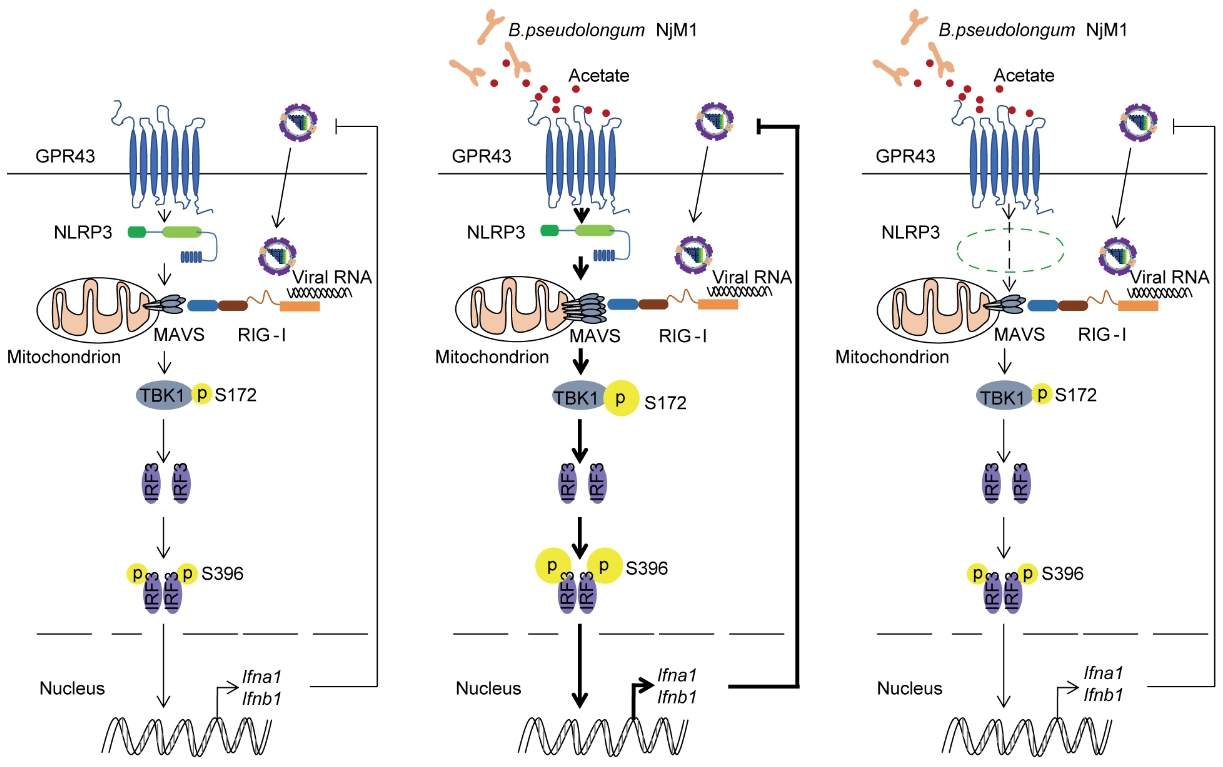
Proposed model for the role of NLRP3 in mediating acetate-enhanced induction of IFN-I. (Image by SIII)
Contact
MENG Guangxun
Shanghai Institute of Immunity and Infection, CAS
E-mail: gxmeng@siii.cas.cn
Reference: https://www.nature.com/articles/s41467-023-36323-4#Abs1

