In the study published in the journal Cancer Cell on September 19, entitled The intratumor mycobiome promotes lung cancer progression via myeloid-derived suppressor cells, Prof. CHEN Changbin’s group at the Shanghai Institute of Immunity and Infection of the Chinese Academy of Sciences reported that intratumor mycobiome, albeit at low biomass, promotes lung cancer progression and could be targeted at the strain level to improve patients with lung adenocarcinoma (LUAD) outcome.
Aspergillus sydowii, the enriched tumor-resident fungus isolated in this study, promotes lung adenocarcinoma progression by inducing myeloid-derived suppressor cells (MDSCs) expansion and activation through IL-1β secretion via β-glucan-mediated Dectin-1/CARD9 pathway. By integrating multiple omics technologies such as deep metagenomics, culturomics, genomics and transcriptomics, the species and distribution characteristics of fungal microbiome in different tissue parts of LUAD patients were analyzed. The paper elucidates the mechanism by which the intratumoral fungus A. sydowii induces the tumor immunosuppressive microenvironment through the interaction with the host, and ultimately promotes the development of lung cancer. These findings identify the intratumor mycobiome, despite their low abundance, promotes tumor progression by inducing an immunosuppressive tumor microenvironment (TME). This research achievement will greatly promote the new strategy of individualized target-immunotherapy for lung cancer based on targeted fungi, and fill the gap in the research on the molecular mechanism of fungi and lung cancer.
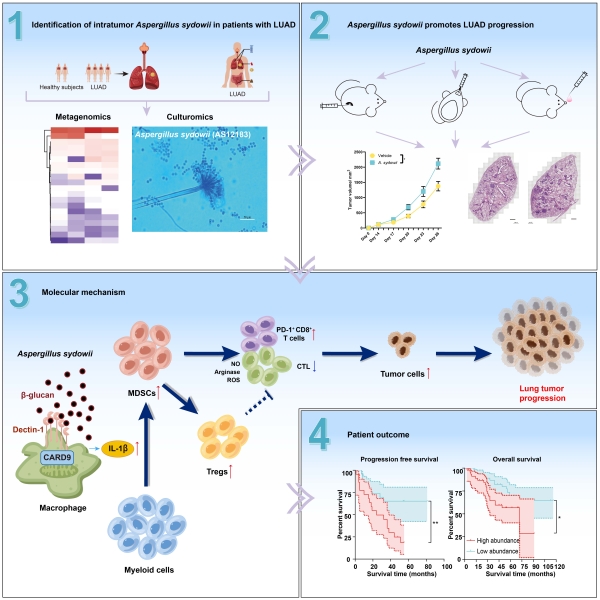
Graphical abstract (Image by SIII)
1. Enriched intratumor A. sydowii is identified in patients with LUAD
2. A. sydowii promotes LUAD in three syngeneic murine lung cancer models
3. A. sydowii induces MDSCs by IL-1b secretion via b-glucan/Dectin-1/CARD9 pathway
4. Enriched A. sydowii is associated with immunosuppression and poor patient outcome
Contact
CHEN Changbin
Shanghai Institute of Immunity and Infection, CAS
E-mail: cbchen@siii.cas.cn
Reference: https://doi.org/10.1016/j.ccell.2023.08.012

