On Dec 1, 2017, The Nature Communications published anArticle research work entitled “Remodeling of the gut microbiota by hyperactive NLRP3 induces regulatory T cells to maintain homeostasis” from Guangxun Meng’s unit, Institute Pasteur of Shanghai, Chinese Academy of Sciences.
Inflammasomes are recently identified cytosolic multi-protein complexes mediating specific proinflammatory signals, such as maturation of IL-1β,IL-18 and processing Gasdermin D to provoke a specialized cell death, pyroptosis. Accumulating data has suggested that multifaceted roles of inflammasomes have been involved in gut homeostasis and inflammatory pathologies. Among many different inflammasomes, NLRP3 stands out for its complicated activation mechanisms, as well as its clinical significance. But the role of NLRP3 inflammasome in gut homeostasis and diseases is not well understood. In humans, dysregulation of inflammasomesignalling by gain-of-function mutations (such as NLRP3-R260W) in the coding region of NLRP3 results in a group of autoinflammatory diseases called cryopyrin-associated periodic syndromes (CAPS). Interestingly, mice carrying the homologous mutation in NLRP3 (NLRP3-R258W) show similar inflammation in skin and joints, but retained a healthy intestine.This phenomenon indicates an unknown pathway for NLRP3-R258W tomaintain gut homeostasis despite its hyperactivity.
Ph.D.candidateXiaomin YAO and Yue XING supervised by Prof. GuangxunMENG, in cooperation with Prof. Liping ZHAO’s lab at Shanghai Jiaotong University school of life sciences, found the NLRP3-R258W mice are not only healthy with their intestines, but also are with stronger resistance to DSS colitis and colorectal cancer; the mutation leads to a distinct gut microbiota with a sparingly interconnected co-abundance network, and nourished functional bacteria capable of inducing Tregs, which mediates neutralization of intestinal inflammation. Lack/inhibition of Tregs will lead to spontaneous colitis/loss of resistance to induced acute colitis. Mechanistically, the microbiota was reshaped via increased local antimicrobial peptides boosted by the enhanced IL-1β but not IL-18 production from lamina propria mononuclear phagocytes containing mutated NLRP3. This study for the first time revealed how NLRP3 functions in the intestine and how it cooperates with gut microbiota to harnessing Tregs to maintain intestinal homeostasis. These findings highlight the importance of NLRP3’s contribution to the gut diseases and homeostasis, providing potential molecular targets for future treatments of intestinal disorders.
This project is funded by grants from Natural Science Foundation of China, National Key Basic Research programs of China, as well as Strategic priority program and International partnership program of Chinese Academy of Sciences.
Links:https://www.nature.com/articles/s41467-017-01917-2
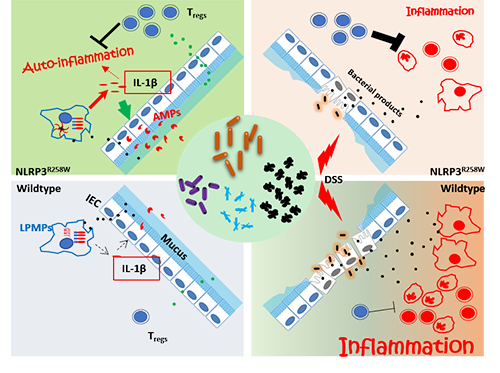
Remodeling of the gut microbiota by hyperactive NLRP3 inflammasome induces regulatory T cells to maintain intestinal homeostasis

