Recently, researchers at Institut Pasteur of Shanghai, Chinese Academy of Sciences have made an important progress in developing prophylactic hepatitis c virus (HCV) vaccine, and published online an article entitled "A trivalent HCV vaccine elicits broad and synergistic polyclonal antibody response in mice and rhesus monkey" in Gut (2016 impact factor 16.6) on November 27. This work was conducted jointly by Dr. Jin Zhong’s group and Dr. Zhong Huang’s group, and was also contributed by Dr. Dongming Zhou at Institut Pasteur of Shanghai and Dr. Qiang Sun at Institute of Neuroscience in Shanghai, Chinese Academy of Sciences.
HCV is one of the major pathogens leading to chronic hepatitis, cirrhosis and liver cancer. About 180 million people worldwide are estimated to be infected by HCV, and the distribution geography of the infection cases is linked to virus genotypes. A new generation of antiviral drugs can effectively cure HCV infection, but their therapeutic application may be impeded by high cost of treatment, potential drug resistance and limited success towards patients with advanced cirrhosis and liver cancer. A prophylactic HCV vaccine is still extremely important for control of hepatitis C epidemic. HCV, classified into seven genotypes and many more subtypes, possesses high genetic variation between different genotypes (about 30% sequence difference), making it difficult to develop an effective HCV vaccine.
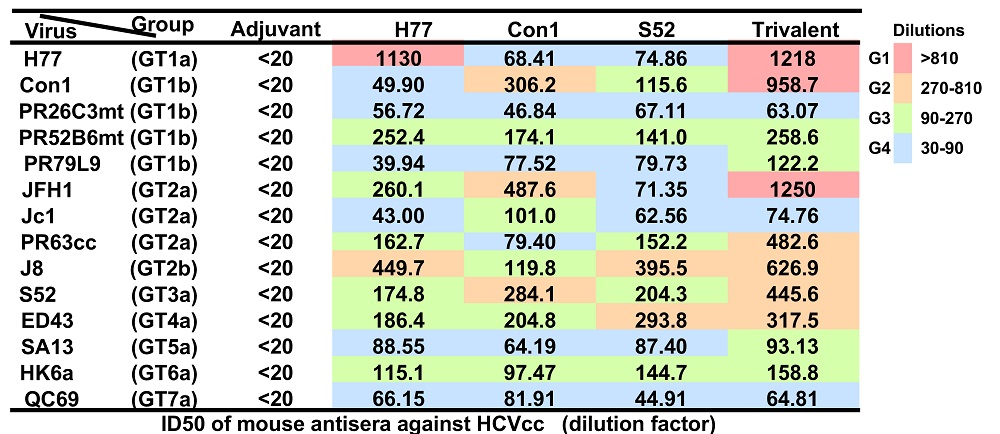
Under the guidance of Prof. Jin Zhong and Zhong Huang, Ph.D students Xuesong Wang and Yu Yan developed a trivalent HCV vaccine consisting of soluble envelope E2 protein (sE2) from H77 strain (genotype 1a), Con1strain (genotype 1b) and S52 strain (genotype 3a), which are most frequently distributed genotypes. This trivalent vaccine induced more efficient and broader neutralizing antibody response compared to three monovalent sE2 vaccines in mice. Further study showed that each sE2 component of the trivalent vaccine can elicit different antibody spectrum that work synergistically to neutralize HCVcc strains Con1 (genotype 1b), JFH1 (genotype 2a), PR63cc (genotype 2a) and S52 (genotype 3a). Furthermore, this trivalent vaccine induced broad neutralizing antibodies with higher efficiency and better uniformity compared to the monovalent vaccine in rhesus macaques. In addition, the trivalent sE2 vaccine induced HCV-specific T cell immune response in rhesus monkeys. In summary, these results demonstrate that the trivalent sE2 subunit vaccine has the advantages of high-yield antigen production, no competition or interference between individual components within the trivalent formulation, and capability of inducing synergistic neutralizing antibodies, which lays important foundations for future commercial development.
This research was supported by Chinese National 973 Research Program from the Ministry of Science and Technology, Key Project of National Natural Science Foundation of China, and International Partnership Program for Creative Research Teams of immunology research of HCV vaccine development of the Chinese academy of sciences.
It is worth mentioning that Dr. Zhong’s group and Dr. Huang’s group at Institut Pasteur of Shanghai have teamed up in pursing HCV vaccine development and made several important progresses in the past years. The results published in Gut as well as previously in Journal of Virology in 2016 and Journal of Infectious Diseases in 2017 demonstrated that the sE2-based subunit HCV vaccine is a promising candidate for further development.
Link for the article:http://gut.bmj.com/content/early/2017/11/26/gutjnl-2017-314870
Neutralization of anti-trivalent sera from mice against 7 genotypes of HCVcc