On 2nd Nov, 2017, Researchers from Institut Pasteur of Shanghai, Chinese Academy of Sciences and Freiburg Universitypublish the recent research achievement in Nature Communications(Article)titled with “Role of influenza A virus NP acetylation on viral growth and replication” (doi:10.1038/s41467-017-01112-3). This study for the first time discovers the acetylation sites on influenza NP proteins and suggests that virus could mimic epigenetic modifications in eukaryotic cells to regulate virus replication and release.
Influenza A virus is a substantial threat to human health.Estimated, seasonal influenza epidemics attack about 10% of the population worldwide, which results in 3-to-5 million severe illness cases and 250,000-to-500,000 deaths. Occasionally, influenza pandemics, such as the latest H1N1 pandemic in 2009, cause millions of deaths all over the world. InfluenzaA virus possesses a large virus gene pool and a wide range of host species (Fig.1). This is why the virus is prevalent for a long history in nature and hard to be eliminated. The existed drugs for influenza A virus is challenged by resistant strains and new drugs discovering is mandatory to improve the treatment.
In this study, researchers identified acetylation sites of influenza A virus NP protein by systemic mass spectrum assay. Importantly, the work highlights that a fine-tuned acetylation is essential for the virus because hyper-acetylation disrupts vRNP complex and inhibits virus replication, while de-acetylation affects virus release and limits the number of mature progenyvirions (Fig.2). It is known that virus assembly and release is technologically difficult to study and is still a mystery in the field. The researchers are able to integrate various biochemistry, virology, genetic and cell culture technologies to unveil the complicated and elaborate process of virus release showing that histone-like epigeneticmodification is essential in influenza A virus replication. Disrupt the acetylation balance could therefore serve as a new strategy to inhibit virus replication in human cells.
This work is funded by the“Stipendienprogrammmit Shanghai und Jiangsu”from the Baden-Württemberg State of Germany,National Natural Science Foundation of China,Youth Innovation Promotion Association CASand others. Dr. Martin Schwemmle from Freiburg University, Dr. Ke Xu from Institut Pasteur of Shanghai, Chinese Academy of Sciences and Dr. Eugene Chin from Health Science Institute are the co-corresponding authors for this research.
Paper link:https://www.nature.com/articles/s41467-017-01112-3
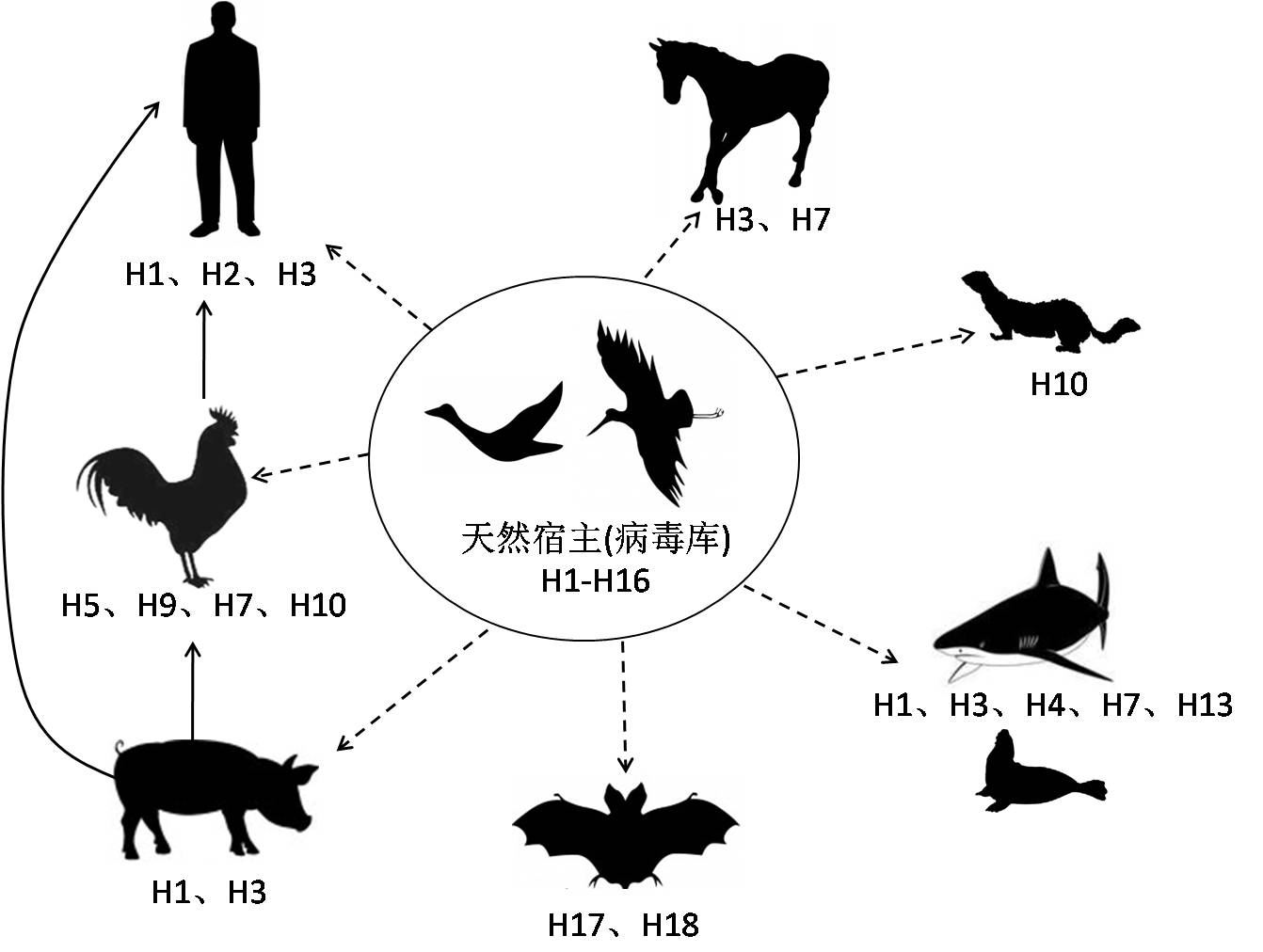
Fig1. Subtypes and cross-species transmission of influenza A virus.
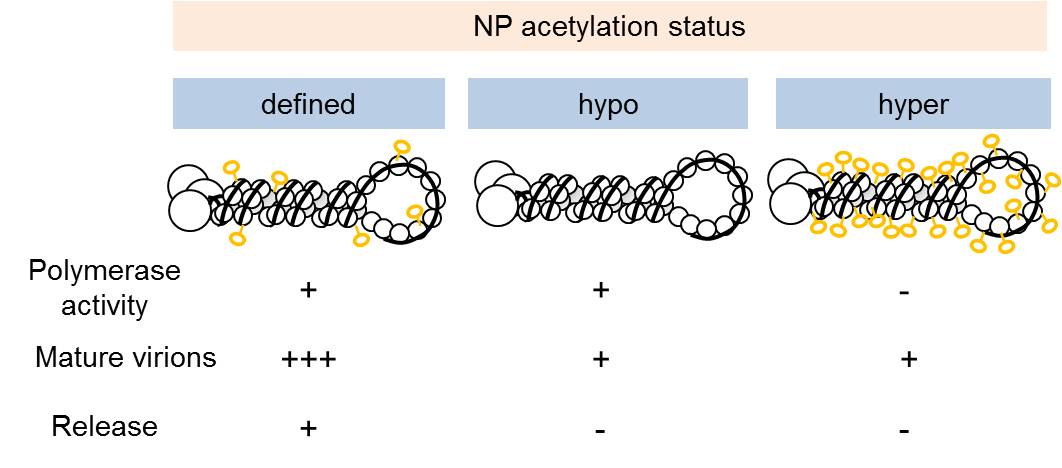
Fig2. Balanced acetylation is essential for virus replication and release.

