On October 15th, the international clinical research journal Journal of Infectious Diseases (JID) published the Phase I clinical trial of candidate HIV vaccines entitled " Vaccination with heterologous HIV envelope sequences and heterologous adenovirus vectors increases T cell responses to conserved regions (HVTN 083)". The research is completed by the largest global HIV vaccine clinical trial group, HIV Vaccine Trials Network (HVTN) of NIH, in collaboration with investigators from 15 US research institutions including Harvard, Columbia, and Rochester Universities, and senior corresponding author Dr Xia Jin, the Head of Viral Disease and Vaccine Translational Research Unit.
An effective vaccine against HIV-1 infection remains an elusive goal and substantialintra-clade, inter-clade viral genetic diversity poses a remarkable challenge for immunogen design. Vaccination with existing vaccination approach often results in high magnitude responses to a small number of immunodominant epitopes, with only weak responses induced against subdominant epitopes.
The Project Chair Dr Jin with Co-chair Dr. Marissa Wilck (University of Pennsylvania) and Dr. Stephen Walsh (Harvard University) gathered 180 healthy volunteers who were randomized into 5 groups receiving combinations of adenovirus vectors (Ad5 or Ad35) and HIV-1 envelope (Env) gene inserts (clade A or B) in a prime-boost regimen. Results showed that heterologous vector regimens had higher numbers of total, EnvA, and EnvB epitopes than homologous vector regimens, and demonstrated a new vaccination strategy that could increase T cell responses to shared epitopes. .
This work is supported by the National Institute of Allergy and Infectious Diseases (NIAID) and the National Center for Advancing Translational Sciences (NCATS).
Details of study can be found online at http://jid.oxfordjournals.org/content/early/2015/10/15/infdis.jiv496.long
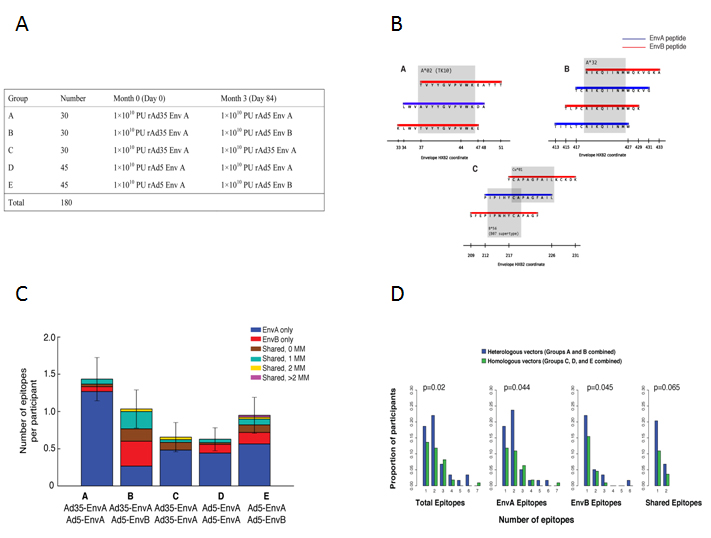
A. Phase I clinical study to assess the safety and tolerability of a heterologous-insert prime-boost vaccine regimen
B. Mapping epitope elicited by the vaccines using overlapping peptides from HIV EnvA and EnvB
C. Mean number of epitopes recognized is 1.38, and high number of epitopes was present in the heterologous vector groups
D. Heterologous vector groups (A & B) had higher number of total, EnvA, and EnvB epitopes recognized

