On October 1, Journal of Biological Chemistry published a research work online entitled “Poly(ADP-ribosyl)ation of FOXP3 Mediated by PARP-1 Regulates the Function of Regulatory T Cells” from a team of researchers led by Bin Li, Institut Pasteur of Shanghai, Chinese Academy of Sciences. This study shows PARP-1 negatively regulates FOXP3 stability and the suppressive function of Regulatory T cells (Treg) at the post-translational level via FOXP3 poly(ADP-ribosyl)ation.
Treg cells are a subpopulation of CD4+ T cells that induce and maintain peripheral tolerance and modulate immune responses. Dysregulation of the development and function of Treg cells has been shown to be associated with many immune-related diseases including autoimmune diseases, chronic inflammation, acute and chronic infection. Forkhead box P3 (FOXP3), known as a key transcription factor of Treg cells, is required for their development, maintenance and function. Studies have shown that the transcriptional activity, stability and localization of FOXP3 are regulated at post-translational levels including acetylation, ubiquitination and phosphorylation. Furthering our understanding on the regulation of FOXP3 stability and its dynamic regulation in Tregs can thus provide therapeutic clues on how to control major inflammatory diseases including autoimmunity and allergic diseases.
Recently, Xuerui Luo, Jia Nie and Shuaiwei Wang supervised by Professor Bin Li at Institut Pasteur of Shanghai, CAS, under the collaboration with professor Hui Hu at the University of Alabama at Birmingham, Birmingham and professor Wanjun Chen at the National Institute of Dental and Craniofacial Research, reveal a novel molecular pathway that how PARP-1 negatively regulates the suppressive function of Treg cells. In this study, they demonstrate that PARP-1 binds to FOXP3 and induces poly(ADP-ribosyl)ation of FOXP3. More importantly, they find that after the treatment with PARP-1 specific inhibitors, the decreased poly(ADP-ribosyl)ation of FOXP3 in Treg cells lead to increased level of FOXP3 by preventing proteasome-mediated degradation, resulting in increased suppressive function of Treg cells. This research suggest that PARP-1 negatively regulates the suppressive function of Treg cells at the post-translational level through FOXP3 poly(ADP-ribosyl)ation. Furthermore, more specific PARP-1 inhibitors will be required both as tools and therapeutics of autoimmune diseases based on the role of PARP-1 in immune system.
This work was supported by Chinese National Program on Key Basic Research Project, National Natural Science Foundation of China, Shanghai Key grant and Shanghai Three-year Plan on Promoting TCM Development.
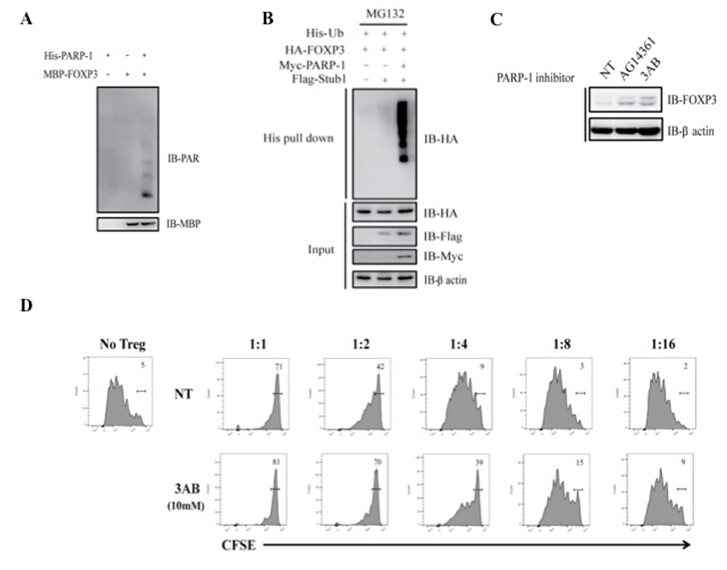
Fig. A: Poly(ADP-ribosl)ation of FOXP3 in vitro. Fig B: PARP-1 promotes poly-ubiquitination of FOXP3. Fig C: PARP-1 inhibitors stabilize FOXP3 protein level. Fig D: PARP-1 inhibitor enhances Treg cells suppressive function.

