On December 26, Journal of Virology published a study entitled “Adenoviral Delivery of Recombinant Hepatitis B Virus (rAd/rHBV) Expressing Foreign Antigenic Epitopes for Immunotherapy of Persistent Viral Infection”. This research was accomplished by Ph.D. student Zhuo Wang with supervision of Dr. Ke Lan and Dr. Qiang Deng at Institut Pasteur of Shanghai, Chinese Academy of Sciences.
Chronic hepatitis B is one of the major public health problems worldwide. Functional impairment of HBV-specific T cell responses has been proved to be the principal reason for host inability to eliminate the persistent infection. In order to treat chronic HBV infection, antiviral immunity has to be reset in favor of a fully activated T-cell response.
Researchers proposed an innovative strategy by using a foreign antigen recombinant HBV (rHBV) as a gene therapy vector. Targeted elimination of wild-type (wt) HBV-infected cells could be achieved by functionally activating an in situ T-cell response against the foreign antigen. To develop this approach more practically viable, Dr. Lan’s group used a recombinant adenovirus (rAd) vector for rHBV delivery. The rAd vector allowed efficient transduction of wtHBV-producing cells, with transferred rHBV undergoing dominant viral replication. Progeny rHBV virions proved to be infectious as demonstrated in primary tupaia hepatocytes. These results widely expanded the antiviral capacity of the replication-defective rAd/rHBV in wtHBV-infected liver tissue. In a mouse model of HBV persistence established by infection with a rAAV virus carrying wtHBV genome, rAd/rHBV-based immunotherapy elicited a foreign antigen-specific T-cell response that triggered effective viral clearance and subsequent seroconversion to HBV. Therefore, it represents an efficient strategy to overcome immune tolerance and eliminate chronic HBV infection.
This work was supported by the National Science and Technology Major Projects and Natural Science Foundation of China.
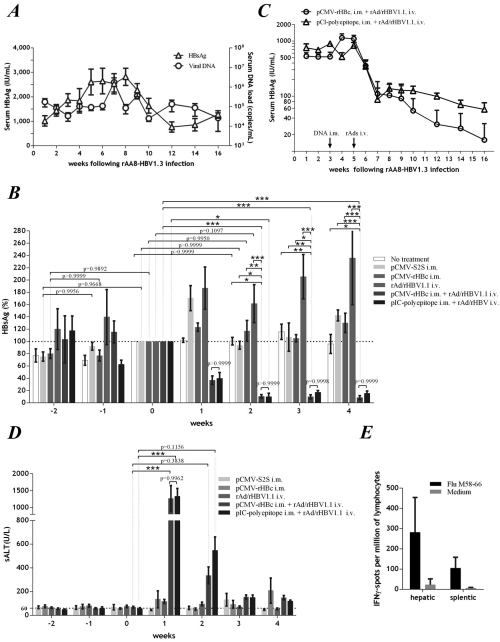
Figure A. HBsAg and viral DNA persistence in rAAV8-HBV infected mice. Figure B, C. Serum HBsAg at indicated time points in rAAV8-HBV infected mice with different immunotherapy strategies. Figure D. sALT activities in rAAV8-HBV infected mice treated as B. Figure E. Elispot assay of rAAV8-HBV infected mice administrated by rAd/rHBV-based active immunotherapy.
Paper link: http://jvi.asm.org/content/early/2013/12/20/JVI.02756-13.abstract

