Regulatory T (nTreg) cells are suppressive cells that control excessive inflammation and mediate immune tolerance. Foxp3 is a crucial transcription factor for Treg differentiation and function. Since inflammation is a regular event in immune effector responses, it may be necessary to disrupt Treg and Foxp3 function to allow for the facilitation of pro-inflammatory triggers.
Recently, CHEN Zuojia From LI Bin’s lab at the CAS Key Laboratory of Molecular Virology and Immunology, Institut Pasteur of Shanghai, CAS, in collaboration with PAN Fan’s lab at Johns Hopkins University School of Medicine, revealed a molecular pathway by which Foxp3 is downregulated at the protein level in response to inflammatory cues, thus allowing for initial robust effector responses that are required for combating imminent threats to the host.
In this study, Foxp3 was found interacting with the E3 Ligase Stub1, induced by inflammatory stimuli (LPS, IL-1a etc.). Stub1 was found mediating the ubiquitination and subsequent degradation of Foxp3. While Foxp3 has been known being posttranslationally modified by its ligation to ubiquitin moieties, the identification of the responsible E3 Ligase has remained elusive. This Stub1-mediated Foxp3 protein downmodulation was further found dependent on the inducible heat-shock protein HSP70, which acts as a co-factor for the interaction between Stub1 and Foxp3. Furthermore, by utilizing both over expression and knock-down approaches, Stub1-mediated Foxp3 degradation had profound effects toward Treg stability and function in vitro and in vivo. In particular, the downregulation of Foxp3 coincided with the loss of suppressive function and the acquisition of a Th1-like effector phenotype in Tregs ectopically expressing Stub1.
These findings may have significant implications for the fields of Treg biology and immune homeostasis. Firstly, while they contribute to mounting observations of instability or flexibility in the Treg lineage, they also provide a mechanistic explanation for the reported tendency of Treg cells to lose Foxp3 expression under conditions such as hyper-inflammation. Importantly, the hitherto unrecognized pathway of Foxp3 regulation characterized in this manuscript may provide opportunities for novel therapeutic interventions aimed at modulating Foxp3 expression. Future exploration and exploitation of this Stub1-dependent regulatory pathway could potentially be used to benefit treatments targeting autoimmune diseases, chronic inflammation, and cancer.
The study entitled“The Ubiquitin Ligase Stub1 Negatively Modulates Regulatory T Cell Suppressive Activity by Promoting Degradation of the Transcription Factor Foxp3“ has been published online in Immunity.
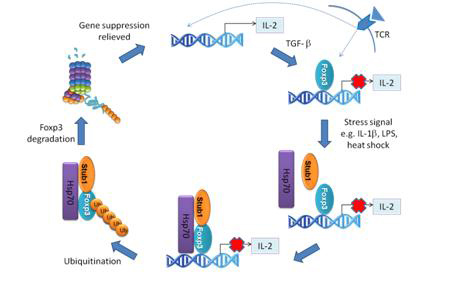
Diagram showing Stub1-mediated downregulation of Foxp3 during inflammatory stress

