Postoperative cognitive decline (PCD) complicates recovery from surgery especially in patients with the Metabolic Syndrome (MetaS). It is recently recognized that neuroinflammatory responses contribute to PCD that subsides when the inflammation resolves, but the exact neural-immunol regulatory mechanisms are not well characterized.
In collaboration with Dr. Mervyn Maze, chair of the Department of Anesthesia and Perioperative Care, University of California San Francisco, the research team led by Professor SU Xiao from Institut Pasteur of Shanghai, Chinese Academy of Sciences utilized a well-validated rat model of MetaS in which rats have been selectively bred using an easy fatigability phenotype (low capacity runners; LCR) to interrogate neural and humoral inflammation-resolving mechanisms for severe PCD.
They found that the isolated splenic mononuclear cells (MNCs) from LCR rats were relatively resistant to cholinergic or adrenergic receptor agonist inhibition of LPS-activated NF-κB and TNF-α generation, respectively, compared to these cells from control high capacity runner (HCR) rats, both before and after surgery. In the spleens of LCR rats, flow cytometry analysis revealed a marked reduction of cells required for neural resolution of inflammation including α7 nicotinic acetylcholine receptor-expressing macrophages, β2 adrenergic receptor-expressing CD3+ lymphocytes (acetylcholine-synthesizing cells), regulatory T cells, and arginase 1-expressing CD11b/c+ M2 macrophages. An increase of inflammatory cytokine IL-6 mRNA and precluded M2 macrophage polarization in the hippocampus of postoperative LCR rats contribute to the persistence of hippocampal inflammation. In LCR rats surgery decreased the anti-inflammatory lipoxin A4 but increased the pro-inflammatory leukotriene B4; diametrically opposite changes were noted in postoperative HCR rats. All of these defects in neural and humoral inflammation-resolving pathways were present at the time that LCR rats exhibited more severe cognitive decline. These findings provide novel strategies for identifying elective surgical MetaS patients at risk and targeting therapeutic interventions postoperatively.
This work was partially supported by Parker B Francis Award (X.S.), the Knowledge Innovation Program of the Chinese Academy of Sciences (Y114P11209, X.S.), the National Natural Science Foundation of China (81270139, X.S.), and startup fund (X.S.) from the Department of Anesthesia, University of California San Francisco.
The Molecular Medicine online reported this result on January 3, 2013. For full text of the paper, please click: http://www.molmed.org/pdfstore/12_351_Su.pdf
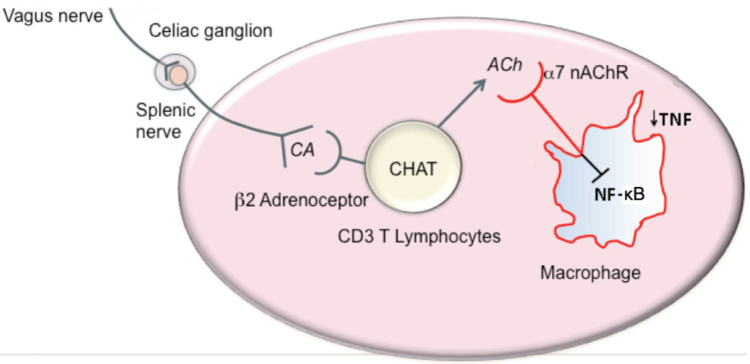
Figure 1. Hypothetic model for cholinergic inflammation-resolving mechanisms

