On January 11, 2012, Journal of Virology published online a research article entitled “A Human Antibody Recognizing a Conserved Epitope of H5 Hemagglutinin Broadly Neutralizes Highly Pathogenic Avian Influenza H5N1 Viruses” by Paul Zhou’s laboratory at the Institut Pasteur of Shanghai, Chinese Academy of Sciences. This is the first report on a human monoclonal antibody that exhibits broad neutralization activity against almost all reported clades and subclades of H5N1 viruses.
Since 1996 highly pathogenic avian influenza (HPAI) H5N1 virus has infected over 500 million poultry and an increasing number of humans in Asia, Europe and Africa. As of October 10, 2011, 566 human H5N1 infections have been confirmed, resulting in 332 deaths (http://www.who.int/csr/disease/avian_influenza/country/en/). Current vaccines against H5N1 virus provide immunity to viral isolates similar to vaccine strains. High affinity neutralizing antibodies against conserved epitopes could provide immunity to diverse influenza strains and protection against future pandemic viruses. In this study, using a highly sensitive H5N1 pseudotype-based neutralization assay to screen human monoclonal antibodies produced by memory B cells from a H5N1 infected individual and molecular cloning techniques, Ph. D. student Hongxing Hu and his colleagues in Professor Paul Zhou’s laboratory developed three fully human monoclonal antibodies. Among them, antibody 65C6 exhibited potent neutralization activity against all H5 clades and subclades except for subclade 7.2 and prophylactic and therapeutic efficacy against highly pathogenic avian influenza H5N1 viruses in mice. Studies on HA-antibody complexes by electron microscopy and epitope mapping indicate that antibody 65C6 binds to a conformational epitope on the tip of the membrane-distal globular domain of HA. Thus, we conclude that antibody 65C6 recognizes a neutralization epitope in the globular head of HA that is conserved among almost all divergent H5N1 influenza stains. On the one hand antibody 65C6 may potentially be used either alone or in combination with antibodies of different binding specificities or with small-molecule inhibitors to treat human cases of various (sub)clades of H5N1 virus infection and on the other hand, immunogens based on this common H5 HA neutralization epitope may be developed to elicit broad neutralizing antibody responses against almost all (sub)clades of H5N1 viruses.
This project was carried out in the collaboration with Professor John Skehel’s laboratory at National Institute for Medical Research, Mill Hill, United Kingdom, Professor Vincent Deubel’s laboratory at the Institute Pasteur in Cambodia and Professor Linqi Zhang’s laboratory at the Tsinghua University. Professor Boping Zhou at the Shenzhen Third Hospital provided precious patient samples. This work was supported by research grants from the French Ministry of Health, the National Natural Science Foundation of China , National Science and Technology Major Project of MOSTand the Li Ka-Shing Foundation of Hong Kong and the UK Medical Research Council.
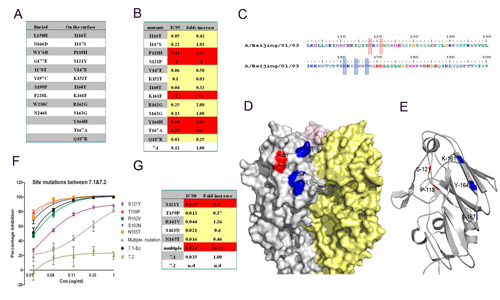
Fig. Determination of Amino acid residues involved in 65C6 neutralization epitope

