In the study published in the journal Journal of Cell Biology on Feb. 18, 2022, entitled Cell migration orchestrates migrasome formation by shaping retraction fibers, Prof. JIU Yaming’s group at Institut Pasteur of Shanghai, the Chinese Academy of Sciences reported that cell migration pattern (persistence and velocity) regulates the formation of migrasome.
Migrasome is a recently discovered vesicle-like cell structure. As cells crawl across the extracellular matrix, retraction fibers (RFs) are pulled out of the plasma membrane at the rear of the cell and then migrasomes form on RFs. It has been reported that migrasome could regulate key cellular physiological processes, including mitochondrial quality control, intercellular communication, and lateral transfer of mRNA and protein.
It is worth noting that the formation of migrasome is strictly dependent on cell migration. However, whether and how different cell migration patterns regulate the formation of migrasome has not been explored.
Here, JIU’s group observed the random migration of single TSPAN4-GFP L929 cells by living cell imaging, and found that there were significantly fewer migrasomes in turning cells compared to straight persistently migrating cells. The major insight underlying this observation is that as the cells elongate, their rear ends become narrower, subsequently resulting in fewer retraction fibers during impersistent migration. The phenomenon was identified by data analysis of indexes related to migrasome and RFs in the turning region and the persistent migration region of sharp turning cells.
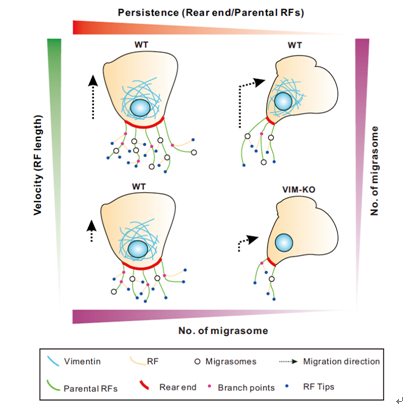
In addition to migration persistence, migration speed was revealed to be positively corelated with migrasome formation by analysis of persistent migrating cells with different migration rates, due to the derived length of retraction fibers. Persistence and speed are critical cell migration parameters for migrasomes formation in other cell types as well, such as human gastric carcinoma cell line MGC803 and normal rat kidney cell line NRK, which proved the universality of migration pattern regulation.
To substantiate the hypothesis, intermediate filaments vimentin was chosen to be a target for cell migration regulator. Genetically removing vimentin compromises cell migration speed and persistence, and leads to fewer migrasomes.
Together, this work shed light on the critical roles of two cell migration patterns, persistence and speed, in the control of migrasome formation by regulating retraction fibers, which provided information for future research of biogenesis of migrasomes. This work was supported by the Chinese Academy of Science, National Natural Science Foundation of China and Ministry of Science and Technology of China, etc.
Contact
JIU Yaming
E-mail: ymjiu@ips.ac.cn
Link of CAS: https://english.cas.cn/newsroom/research_news/life/202202/t20220218_300954.shtml

