The SARS-CoV-2 spike protein is responsible for host receptor recognition, membrane fusion and viral infection. Understanding the molecular and cellular mechanisms of spike-driven viral entry is a research priority in curbing the ongoing pandemic and preventing future coronavirus outbreaks.
In a study entitled SARS-CoV-2 spike engagement of ACE2 primes S2′ site cleavage and fusion initiationpublished in the journal PNAS on December 21, 2021, Prof. MENG Guangxun and Prof. Lavillette Dimitri’s groups at the Institut Pasteur of Shanghai of the Chinese Academy of Sciences, reported new mechanistic insights into the activation of SARS-CoV-2 spike protein.
The researchers unveiled a cellular event responsible for the activation of SARS-CoV-2 spike and the generation of S2’. Using multiple experimental models, they demonstrated that the proteolytic event occurs within the spike S2 subunit and is a molecular switch coupled to membrane fusion and viral infection.
Besides, the researchers showed that after host receptor recognition of angiotensin-converting enzyme 2 (ACE2), spike-driven syncytia formation requires the presence of S2’ cleavage site at arginine 815, but not the furin cleavage site at 685. Point mutations of the S2’ cleavage site is not only effective against the original spike, but also shown to be essential for the Alpha, Beta and Delta variants of concern.
Hence, targeting spike proteolytic event at the S2’ may serve as a potential antiviral option against the current SARS-CoV-2 outbreak and future cross-strain coronavirus infections.
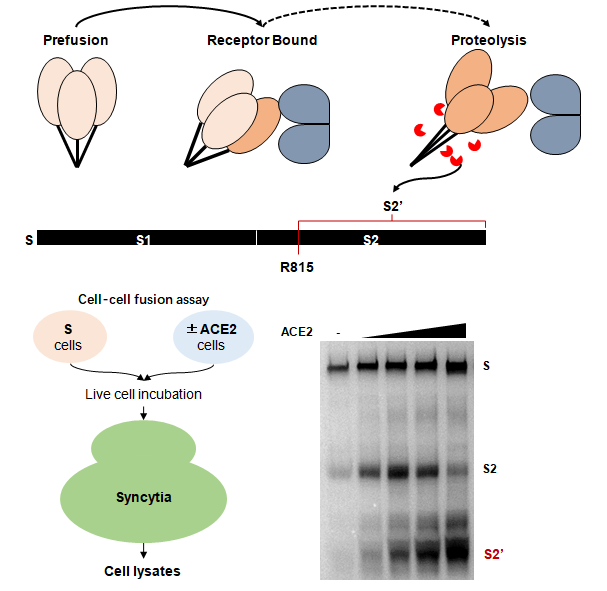
Schematics of spike activation by receptor ACE2 and the downstream proteolytic cleavage at the S2’. (Image by IPS)
Contact:
MENG Guangxun
E-mail: gxmeng@ips.ac.cn
Reference: https://www.pnas.org/content/119/1/e2111199119
Link of CAS: https://english.cas.cn/newsroom/research_news/life/202112/t20211227_295216.shtml

