The researchers from Dr. ZHONG Jin’s group at Institut Pasteur of Shanghai of the Chinese Academy of Sciences reported a new finding on the effect of glycometabolism on HCV release and its potential impact on the viral evasion of host immune response. This study was published online in PLOS Pathogens on July 23, 2021.
Hepatitis C virus (HCV) is a positive-stranded RNA virus that causes chronic hepatitis, cirrhosis, and hepatocellular carcinoma. HCV infectious cycle comprises viral entry, uncoating, translation and replication of viral RNA, assembly into new virions and release. Establishment of HCV cell culture system (HCVcc) has yielded many insights into complete HCV infectious cycle in Huh7 cell and Huh7-derived human hepatoma cell lines.
However, due to metabolic reprogramming, tumor cells and normal cells vary a lot in many metabolic pathways including glycometabolism. Unlike normal cells, tumor cells usually undergo a high rate of glycolysis instead of oxidative phosphorylation for energy production even in the presence of sufficient oxygen. This aerobic glycolysis in tumor cells is referred to as the “Warburg effect”.
It has been reported that tumor cells grown in galactose-containing medium are forced to change their energy metabolism from aerobic glycolysis to oxidative phosphorylation.Therefore, exploring HCV infectious cycle in galactose culture system may provide clues about HCV natural infection in vivo.
In this study, the researchers found that when Huh7 cells are cultured in galactose medium, HCV release, but not viral entry, genome replication or virion assembly, is significantly blocked. Further study showed that intracellular infectious virions are trapped in multivesicular bodies (MVBs) in the cells and cannot be released. Blockade of the MVB-lysosome fusion or treatment with proinflammatory cytokines promotes HCV release in the galactose medium-cultured cells. Mechanistic study showed that this glycometabolic regulation of HCV release is mediated by MAPK-p38 phosphorylation.
Finally, the researchers found that HCV cell-to-cell transmission is not affected by glycometabolism, suggesting that HCV cell-to-supernatant release and cell-to-cell transmission are two mechanistically distinct pathways. Based on above results, the researchers proposed a new concept that HCV may exploit metabolic state in hepatocytes to favor its spread through the cell-to-cell transmission in vivo, with a low efficiency of releasing into circulation. This strategy would allow HCV to effectively evade antibody neutralization and host immune surveillance to establish persistent infection. In general, this study provides new insights into the relationship of glycometabolism and HCV release and HCV natural infection process.
The work was completed by the ZHONG Jin Lab at Institut Pasteur of Shanghai of the Chinese Academy of Sciences, in collaboration with Dr. HE Yongning’s group at the CAS Center for Excellence in Molecular Cell Science and Dr. JIU Yaming’s group at Institut Pasteur of Shanghai. Dr. YU Tao, PhD student YANG Qiankun and TIAN Fangling are the co-first authors of the paper. This study was supported by the grants from Strategic Priority Research Program of the Chinese Academy of Sciences and the National Natural Science Foundation of China.
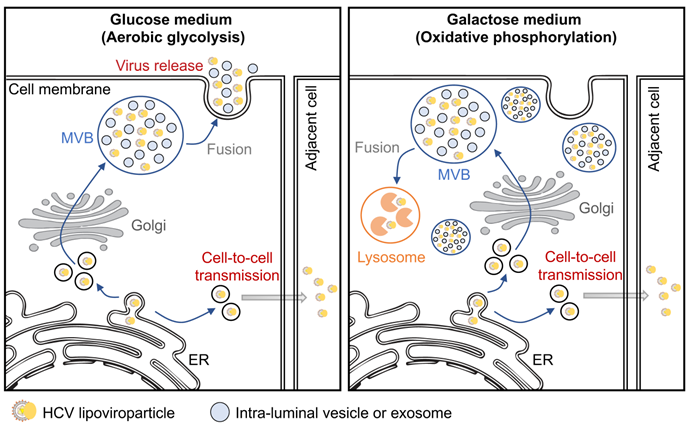
Working model of glycometabolism regulating HCV release. (Image by IPS)
Contact
ZHONG Jin
E-mail: jzhong@ips.ac.cn
Article link:
https://journals.plos.org/plospathogens/article?id=10.1371/journal.ppat.1009746
Link of CAS:
https://english.cas.cn/newsroom/research_news/life/202107/t20210726_276248.shtml

