On January 11, the Nature Press journal Nature Communications published the research article “Development and structural basis of a two-MAb cocktail for treating SARS-CoV-2 infections”. This study was led by Prof. HUANG Zhong’s research group at Institut Pasteur of Shanghai of the Chinese Academy of Sciences in collaboration with Prof. CONG Yao at Center for Excellence in Molecular Cell Science of Chinese Academy of Sciences as well as Prof. XIE Youhua and Prof. DENG Qiang at Shanghai Medical College of Fudan University.
The ongoing pandemic of coronavirus disease 2019 (COVID-19) is caused by a newly identified coronavirus named severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Individuals infected with SARS-CoV-2 may develop severe respiratory manifestations and even death. Neutralizing antibodies play a major role in the antiviral immunity and have been shown to be a viable option for developing therapies against viral infections.
Previous work by Prof. HUANG Zhong’s group demonstrated that immunization with the receptor-binding domain (RBD) of SARS-CoV-2 elicits high levels of neutralizing antibodies against SARS-CoV-2 (Zang et al. Cell Discovery 2020, doi: 10.1038/s41421-020-00199-1). In this study, researchers isolated two groups of mouse neutralizing monoclonal antibodies (MAbs) targeting the RBD on the SARS-CoV-2 spike (S) from RBD-immunized mice. MAbs 2H2 and 3C1, representing the two antibody groups, respectively, bind distinct epitopes and are compatible in formulating a noncompeting antibody cocktail.
A humanized version of the 2H2/3C1 cocktail is found to potently neutralize authentic SARS-CoV-2 infection in vitro with half inhibitory concentration (IC50) of 12 ng/mL and effectively treat SARS-CoV-2-infected mice even when administered at as late as 24 h post-infection. They determine an ensemble of cryo-EM structures of 2H2 or 3C1 Fab in complex with the S trimer up to 3.8 ? resolution, revealing the conformational space of the antigen-antibody complexes and MAb-triggered stepwise allosteric rearrangements of the S trimer, delineating a previously uncharacterized dynamic process of coordinated binding of neutralizing antibodies to the trimeric S protein. Their findings provide important information for the development of MAb-based drugs for preventing and treating SARS-CoV-2 infections.
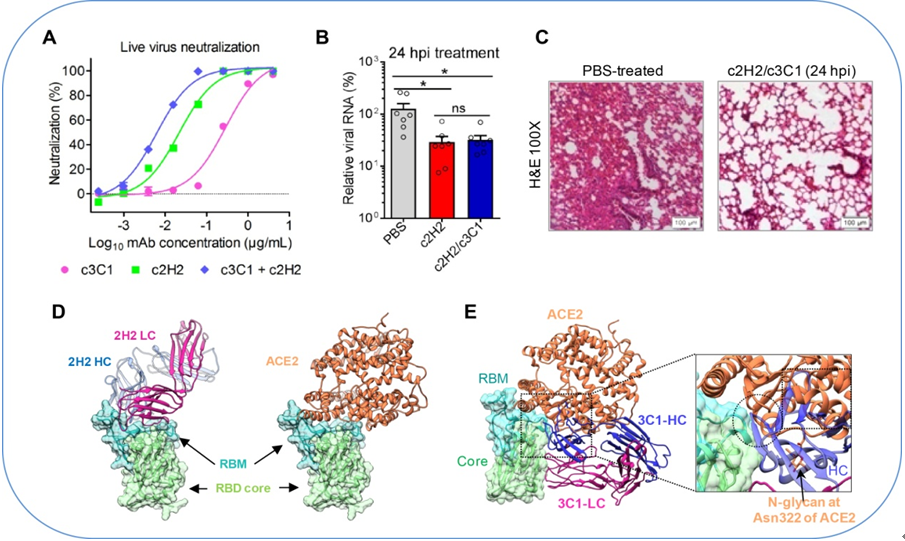
A. Neutralization activity of the human-mouse chimeric MAb cocktail against authentic SARS-CoV-2.
B-C. In vivo therapeutic efficacy of the c2H2/c3C1 cocktail against SARS-CoV-2 infection. B. qRT-PCR analysis of viral RNA copies present in lung tissues after 3 days of infection. C. H&E staining of lung tissue sections at 3 days post infection.
D. 2H2 Fab (left) and ACE2 (right) share overlapping epitopes on RBD and would clash upon binding to the S trimer.
E. ACE2 would clash with the heavy chain of 3C1 Fab (blue). They share overlapping epitopes on the RBD. (Image by IPS)

