On April 1, the Journal of Clinic Investigation published the research article “Gut epithelial TSC1/mTOR controls RIPK3-dependent necroptosis in intestinal inflammation and cancer”. This study led by Dr. Hui Xiao’s research group of the Center for Microbiota, Development and Health at Institut Pasteur of Shanghai, Chinese Academy of Sciences, in collaboration with Dr. Huabin Li’s lab at Fudan University School of Medicine. This study demonstrated that the metabolic mTOR pathway has a central role in the sensing and integration of environmental signals from diet and microbiota for the control of RIPK3-dependent cell death, and deregulation of mTOR and RIPK3 pathways by high-fat/high-protein diet contributes to intestinal inflammation and cancer, providing new insight into the pathogenesis of inflammatory bowel disease (IBD) and the development of therapeutic approaches for IBD and colorectal cancer.
The pathogenesis of IBD is multifactorial, involving a diversity of genetic risk genes and various environmental factors. However, the principles underlying the complex interaction between the host and environment factors remain obscure and the treatment for IBD is limited. As such, despite the Western diet and dysbiosis being considered as the most prominent environmental factors associated with IBD, their corresponding host cellular pathways are still poorly defined. Through genetic approaches, Dr. Xiao’s team revealed a surprising role for the TSC1/mTOR pathway, which has been traditionally linked to development, cell metabolism and growth, in the regulation of RIPK3/MLKL-dependent cell death (aka necroptosis) in the gut. Consistently, high-protein or high-fat diet exacerbated RIPK3 expression and activation through the hyperactivation of mTOR, whereas microbiota-depletion diminished mTOR activation and RIPK3-dependent necroptosis. Evidence from human IBD patients and in vivo colitis/colon cancer models collectively suggest that the epithelial TSC1/mTOR/RIPK3 pathway is instrumental in the regulation of intestinal inflammation and tumorigenesis. Importantly, through the sensing and integration of dietary and microbial signals in the gut, the metabolic sensor mTOR functions as a rheostat for the E3 ubiquitin ligase TRIM11, which was found to control RIPK3 ubiquitination and degradation in this study. Overall, the findings of this study add metabolic mTOR pathway as a new twist into the regulation of necroptosis and IBD, provide unique insights into the understanding of how the host responds to the dietary and microbiota cues, and suggest promising therapeutic approaches for IBD and colorectal cancer.
Yadong Xie, a Ph.D. graduated from the University of Chinese Academy of Sciences, and currently a postdoctoral fellow at Fudan University School of Medicine, is the first author of this study; Yifan Zhao, an M.S. graduated from the University of Chinese Academy of Sciences, is the second author of this study. This study was supported by National Natural Science Foundation of China, the Key development and research project, the Chinese Academy of Sciences, and the Shanghai Municipal Science and Technology.
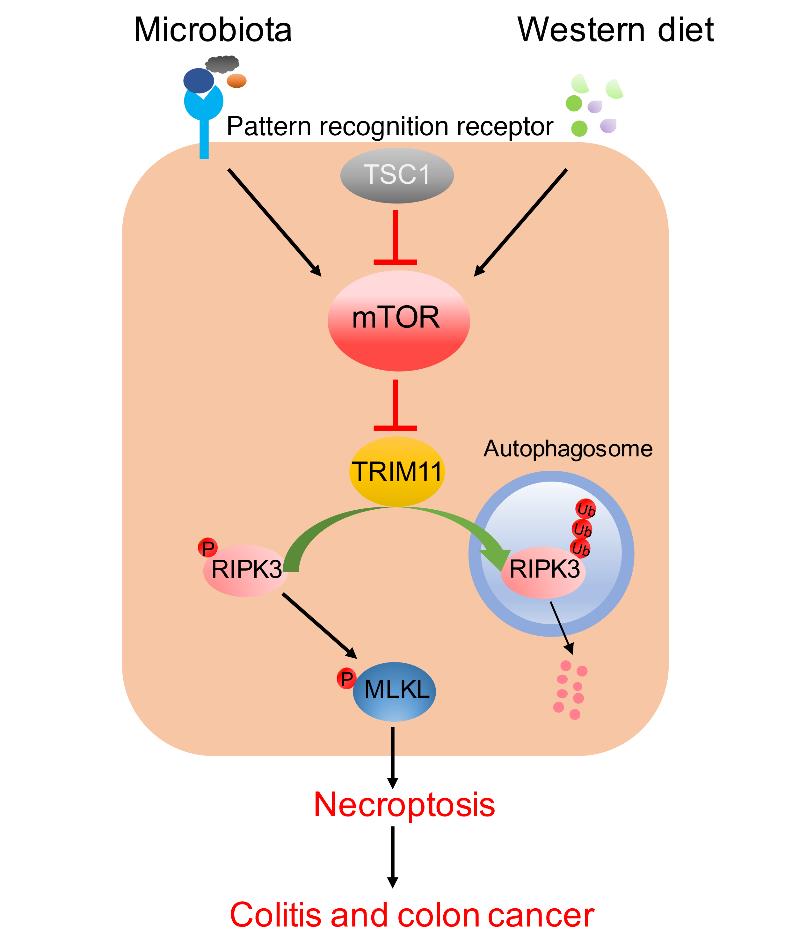
The mTOR pathway integrates the environmental signals from the microbiota and diet to regulate the TRIM11-RIPK3-MLKL axis in the control of IBD and colorectal cancer.
Article link: https://www.jci.org/articles/view/133264

