Researchers at Institut Pasteur of Shanghai identify a novel molecular pathway for the Th17 cells
The Journal of Biological Chemistry (JBC) published online the research conductedby Li Bin from Institut Pasteur of Shanghai, CAS and Lv Lin from rheumatism department of Fudan university Huashan hospital: TRAF5 Mediated K63-linked Polyubiquitination Plays an Essential Role in the Positive regulation of RORγt on promoting IL-17A Expression on October 9th. This study discovered the ubiquitin ligase E3 TRAF5 mediated Th17 cell functional regulation thanks to the cooperation of basic and clinical research.
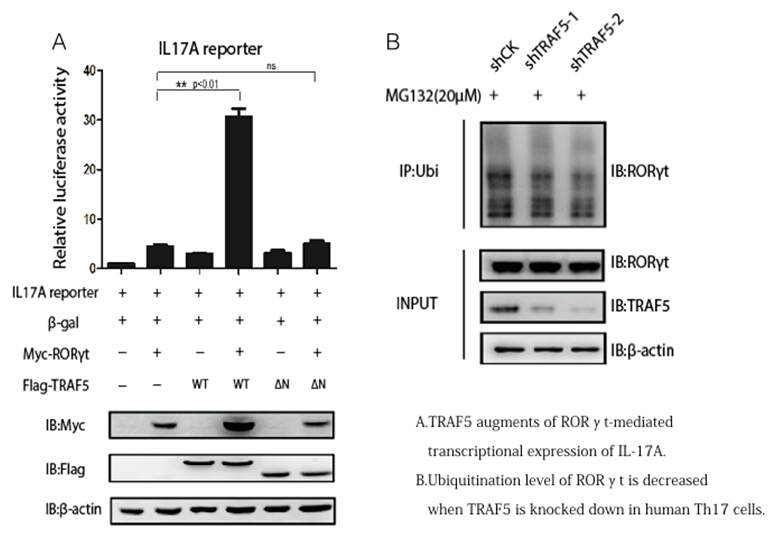
Th17 cells, which have attracted widespread attention as a subset of CD4+ T cells, contributes to the pathogenesis of multiple autoimmune and inflammatory diseases such as systemic lupus erythematosus, rheumatoid arthritis and ankylosing spondylitis by secreting IL-17A, IL-17F and other inflammatory factors. The differentiation of Th17 cells dependson TCR signaling activation and co-simulation withTGF-β and IL-6. In human, the combination of IL-1β and IL-6 or IL-1β and IL-23 could also be effective in inducing naive T cells into Th17 cell. The transcriptional regulatory network research of Th17 cell differentiation is quite wide, however, these aforementioned studies didn’t focus on the ubiquitination and deubiquitination of RORγt, which is considered as a meaningful post- transcriptional modification.
Under the guidance of Principal Investigator Li Bin and Chief Physician Lv Lin, PhD Yang Jing from immune molecule group of Institut Pasteur of Shanghai, master studentWang Xiuwen from rheumatism department of Fudan university Huashan hospital and theircolleagues showed that tumor necrosis factor receptor associated factor 5 (TRAF5), known as an E3 ubiquitin-protein ligase and signal transducer, interacts with and ubiquitinatesRORγt via K63-linked polyubiquitination. And depletion of TRAF5 in Th17 cells destabilizes RORγt protein and down-regulates Th17-related genes. Moreover, up-regulation of TRAF5 mRNA level was found in SLE patient CD4+ T cells. The study not only shed lights on the regulation of Th17 lineage by ubiquitination but also provided new explanation on the role of Th17 cells and potential therapeutic targets towards autoimmune diseases.
The research is supported by Chinese National Program on Key Basic Reasearch Project, Natural Science Foundation of China, Shanghai Key grant and Shanghai Three-year Plan on Promoting TCM Development.