Activity of the forkhead family transcription factor FOXP3 determines the immune function of FOXP3+Regulatory T cells (Tregs). Upon infection or other inflammatory conditions, FOXP3+Tregs may suppress effector immune cell responses leading to the failure of clearing infection or autoimmunity. FOXP3+Tregs may also help to limit collateral tissue damage during heightened inflammation. How Treg cells sense inflammation and shut down its immune suppressive activity to allow immune activation to occur remains largely unknown. Furthering our understanding on the regulation of FOXP3 stability and its dynamic ensemble of enzymatic cofactors in Tregs can thus provide therapeutic clues on how to control major inflammatory diseases including autoimmunity and allergic diseases.
Recently, YayiGao and Jiayou Tang supervised by Professor Bin Li at Institut Pasteur of Shanghai, CAS, and Professor Song-Guo Zheng at Penn State University Hershey College of Medicine, revealed a new molecular pathway by which FOXP3 is downregulated at the protein level in response to inflammatory cues via the activity of an adaptor protein called Deleted in breast cancer 1 (DBC1) which could promote FOXP3 degradation.
YayiGao purified FOXP3 transcriptional complex in FOXP3 stable expressing T cells by tandem affinity purification, and found that Deleted in Breast Cancer 1 (DBC1) is a previously unidentified subunit of FOXP3 complex. Researchers observed that the linker region between the leucine zipper motif and the forkhead domain of FOXP3 interacts with the N-terminal 200 amino acid region of DBC1. Knockdown of DBC1 in FOXP3+Treg cells prevented caspase 8-mediated degradation of FOXP3 under inflammatory cytokine stimulation. Moreover, Jiayou Tang demonstrated that more Treg cells existed in Dbc1-/- mice, and Dbc1-/- FOXP3+Treg cells are more functionally potent compared to Treg cells in wild type mice. This research propose that DBC1 may act as a negative regulator of FOXP3 by attenuating FOXP3 protein stability and activity under inflammation, which could represent a novel molecular pathway for therapeutically modulating FOXP3+Treg function.
The study entitled “Inflammation negatively regulates FOXP3 and regulatory T-cell function via DBC1” was published online in PNAS on June 10, 2015.
This work was supported by grants from Ministry of Science and Technology of China, and National Natural Science Foundation of China.
Paper link: http://www.pnas.org/content/early/2015/06/08/1421463112
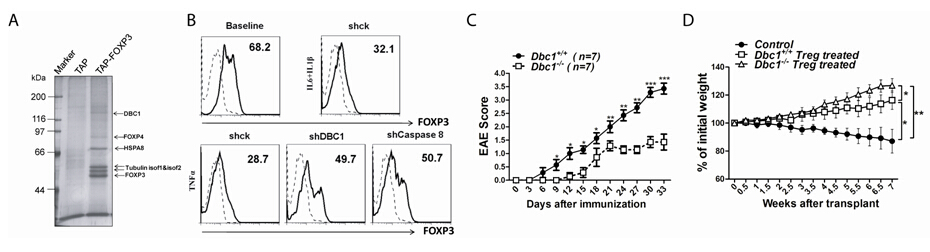
Fig. A. FOXP3 complex were purified by tandem affinity purification in T cells.
Fig. B. DBC1 knockdown or Casapase 8 knockdown human Treg cells maintain more FOXP3 proteins.
Fig.C.Dbc1-/- mice develop less severe autoimmune disease symptoms during EAE induction.
Fig.D.Dbc1-/-Treg cells function profoundly in preventing colitis.

