On August 5, the international academic journal Journal of Biological Chemistry published a research article entitled“PIM1 Kinase Phosphorylates the Human Transcription Factor FOXP3 at Serine 422 to Negatively Regulate itsActivity under Inflammation” from the Unit of Molecular Immunology, Institut Pasteur of Shanghai, Chinese Academy of Sciences.
The project was supervised by Prof. Bin Li in collaboration with Dr. Yu Zhang at Shanghai Jiaotong University. It was primarily executed by Ph.D. student Zhiyuan Li at the Unit of Molecular Immunology. This article reveals a new mechanism of how the immunosuppressive activity of Treg cells is modulated under inflammatory conditions. The finding provides a potential promising target to improve Treg function for treatment of autoimmune diseases, infectious diseases, allergy and tissue rejection.
FOXP3+ Treg cells play a central role in maintaining balance betweentolerance andimmune activation. The transcription factor FOXP3 is considered as a master regulator for the development and function of Treg cells. However, human Treg cells may be unstable and lose FOXP3 expression or convert into effector T cells under inflammatory conditions. Molecular mechanism of Treg cell instability under inflammation remains largely unclear. Protein phosphorylation is the most ubiquitous post-translational modification, and plays important role in most biological processes. Identifying site-specific phosphorylated substrates is fundamental to understand the molecular mechanisms of phosphorylation. It is attractive to study the molecular mechanism of how the activity of human Treg cells can be modulated by phosphorylation.
FOXP3 was found to interact with PIM1 kinase which could phosphorylate human FOXP3 at its Ser422 residue. This phosphorylation down-regulates FOXP3 chromatin binding propensity and its transcriptional regulation activity. The inflammatory cytokine IL-6 was also found to induce PIM1 expression in in vitro expanded Treg cells, while TCR signaling represses its expression significantly. Knockdown of PIM1 or using a PIM1 specific inhibitor would significantly enhance the immunosuppressive activity of in vitro expanded human Tregs. These findings suggest that PIM1 could represent a potential promising target for improving Treg function to prevent autoimmune diseases and tissue rejection under inflammation.
Link of the article:http://www.jbc.org/content/early/2014/08/05/jbc.M114.586651.abstract
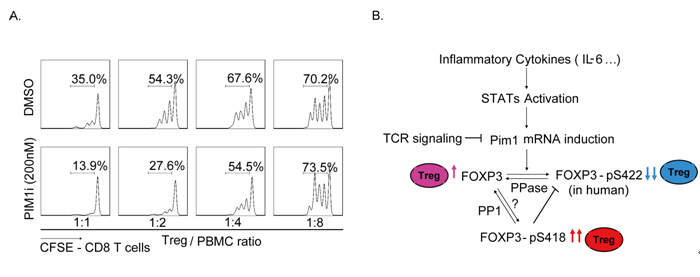
FIGURE: PIM1 kinase phosphorylates the human transcription factor FOXP3 at serine 422 to negatively regulate its activity A. PIM1 specific inhibitor enhances the capacity of the Treg cells to suppress the proliferation of CD8+ T cells; B. Proposed working model for the mechanism of PIM1 regulation of human FOXP3 and Treg activity.

