On July 11, 2014, the International academic journal PLOS Pathogens published an article online entitled “Oncogenic Herpesvirus KSHV Hijacks BMP-Smad1-Id Signaling to Promote Tumorigenesis” by Prof. Ke Lan’s Lab at Institut Pasteur of Shanghai, Chinese Academy of Sciences.
Kaposi’s Sarcoma Associated Herpesvirus (KSHV) is closely related to the development of Kaposi’s sarcoma (KS), primary effusion lymphoma (PEL) and multicentric Castleman’s diseases (MCD). In previous studies, the researchers uncovered the novel roles of Latency Associated Nuclear Antigen (LANA) encoded by KSHV during latency in promoting cell proliferation via stabilizing intracellular Notch1 (Lan, et al. J Virol, 2005; Lan, et al. PNAS 2007). In addition, LANA functions in inhibiting the degradation of Hey1, a major downstream effector of Notch signaling pathway, and is responsible for pathological angiogenesis of KS (Wang, et al. Cancer Res, 2014).
In order to further explore the functions and mechanisms of LANA in KSHV-induced tumorigenesis, the research assistant Deguang Liang, Hao Hu and other group members identified Smad1, a key signaling transducer of BMP pathway, as a novel binding protein of LANA by tandem affinity purification (TAP) under the guidance of Prof. Ke Lan and Prof. Shoujiang Gao from University of Southern California. The researchers confirmed Smad1 MH2 domain at C terminal is responsible for LANA binding and the interaction sustains BMP-activated p-Smad1 in the nucleus, which significantly up-regulates the expression of Ids. In mechanism, LANA enhances p-Smad1 loading on the Id promoter and regulates Id1 as well as Id2 and Id3 at transcription level.
Id family is significantly up-regulated in many types of cancers and promotes tumorigenesis via various mechanisms. In order to verify its role in KS development, researchers firstly confirmed Ids are abundantly expressed in human KS lesions. In parallel, they validated the phenotype in a newly constructed KSHV-transformed cell model (KMM cells) (Jones, et al. JCI, 2012). By genetic inhibition of Id1, the tumorigenic ability of KMM cells is strongly impaired. Collectively, the findings illustrate a novel mechanism by which KSHV hijacks and converts BMP-Smad1-Id cascade into an indispensable oncogenic pathway for tumorigenesis.
To ask for the possibility of utilizing BMP-Smad1-Id as therapeutic target to cure KS, researchers exploited two different kinds of BMP inhibitor, Dorsomorphin and WSS25 to treat KMM cells, finding that they could significantly inhibit the anchorage-independent cell growth of KMM cells in soft agar. Importantly, compared to MM cells, Dorsomorphin shows preferential toxicity to KMM cells. Nevertheless, ectopic expression of Id1 in KMM cells could to some extent rescue the toxicity imposed by Dorsomorphin. Impressively, single treatment with Dorsomorphin was sufficient to significantly inhibit the tumor growth of KMM cells in vivo. In a summary, the finding shows BMP-Smad1-Id pathway could serve as a potential therapeutic target for KS.
The study was supported by the Key Project of Natural Science Foundation of China, the National Basic Research Program (973), etc.
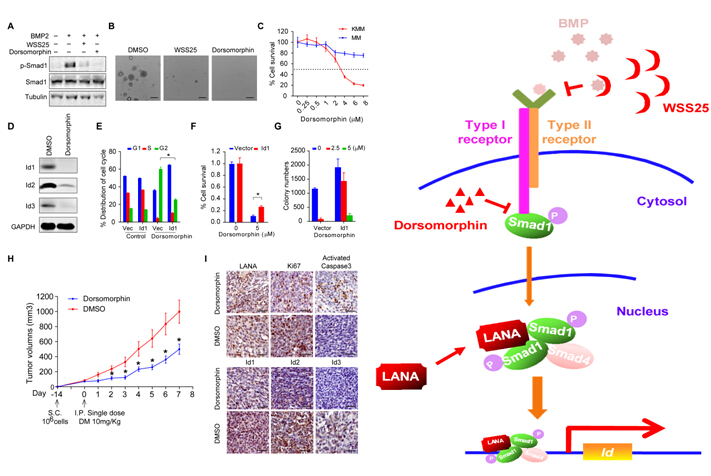
Figure (Left): A-I: Dorsomorphin inhibited tumorigenesis of KMM cells mainly through targeting BMP-Smad1-Id pathway in vitro and in vivo; Figure (Right): A schematic working model.

