On November 21, Journal of Hepatology published an article online entitled “MDA5 Plays a Critical Role in Interferon Response during Hepatitis C Virus Infection” from the Unit of Viral Hepatitis, Institut Pasteur of Shanghai, Chinese Academy of Sciences.
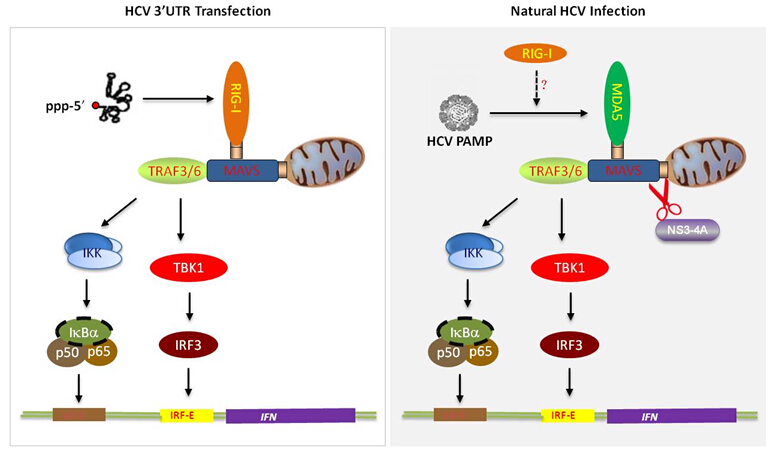
Hepatitis C virus (HCV) is an important human pathogen that can evade host immunity to cause persistent infection, leading to liver cirrhosis and hepatocellular carcinoma. Virus infection can be sensed by host pattern recognition receptors (PRRs) to activate innate immune response against virus infection. Based on the evidence that the transfected 3’UTR of HCV genomic RNA can be recognized by RIG-I to activate interferon production in hepatocytes, it has been believed that the PRR for HCV is RIG-I, a member in the RIG-I like receptor (RLR) helicase family, However, it is difficult to demonstrate the interferon response triggered by RIG-I recognition of the HCV 3’UTR RNA in the context of HCV infection because HCV can shut down the interferon response by using the viral NS3-4A protease to cleave MAVS, a key adaptor molecule essential for the interferon signaling activation. Therefore, it remains elusive what is the real PRR for sensing the virus in the HCV-infected hepatocytes.
Ph.D. students Xuezhi Cao, Qiang Ding and their colleagues in Prof. Jin Zhong’s laboratory at Institut Pasteur of Shanghai, Chinese Academy of Sciences, generated a new hepatic cell line by expressing the genetically engineered MAVS protein, in which HCV infection can activate robust interferon response. They found that RIG-I is not the key PRR to recognize HCV infection. By using the knockout technology, biochemical and functional assays, they found that MDA5, another member of the RLR helicase family is the key PRR during HCV infection. This study provides a new model to investigate how HCV triggers host innate immune response, and identified the real PRR during HCV infection, which should pave the way to explore the molecular mechanisms underlying HCV evasion of host immunity to establish persistent infection.
This study was supported by grants from National Natural Science Foundation of China and Chinese National Science and Technology Mega Projects on Major Infectious Diseases.
Link for this article: http://www.ncbi.nlm.nih.gov/pubmed/25463548