On October 16, The Journal of Biological Chemistry published a study entitled Negative Regulation of Interferon-Induced Transmembrane Protein 3 by SET7-mediated Lysine Monomethylation from the Key Laboratory of Molecular Virology and Immunology at the Institut Pasteur of Shanghai, Chinese Academy of Sciences (CAS) .The researchers led by Prof. Bin Li at Institut Pasteur of Shanghai found that IFITM3 could be monomethylated at its K88 residue to negatively affect its function. Moreover, they discovered that the degree of monomethylation was differentially modulated by viral/host-induced pathways. The exciting finding of the study is the new role of protein lysine methylation in controlling the antiviral activity of host restriction factors.
Zhao Shan, a MS-Ph.D. student supervised by Drs. Bin LI and Andy TSUN at the Unit of Molecular Immunology, performed tandem-affinity purification of IFITM3 followed by mass spectrometry analysis (MS) to identify novel interaction partners and posttranslational modifications of IFITM3. Based on these MS results, they found that lysine-88 of IFITM3 (IFITM3-K88me1) was subjected to monomethylation. By a candidate-based approach, they found that the level of IFITM3-K88me1 was controlled by the methyltransferase SET7. Through overexpression and knockdown studies of SET7, IFITM3-K88me1 was found reduced in its antiviral activity towards VSV infection compared to its non-monomethylated form. Interestingly, monomethylation was promoted by viral infection, but reduced upon interferon-a treatment, indicating that viruses could utilize this SET7 pathway for their own benefit. Finally, they found that the antiviral effects of interferon-α-stimulated genes against VSV and flu infection were also affected by SET7 in an IFITM-dependent manner.
This work will inspire investigators to further study the roles of dynamic posttranslational modifications in determining protein function, and the association between protein methylation and host-pathogen interactions should provide a broad interest to the scientific community.
The research was supported by NSFC, NIH-NSFC, Shanghai ‘Rising Star’ program, Novo Nordisk-Chinese Academy of Sciences Foundation and National Science and Technology Major Project.
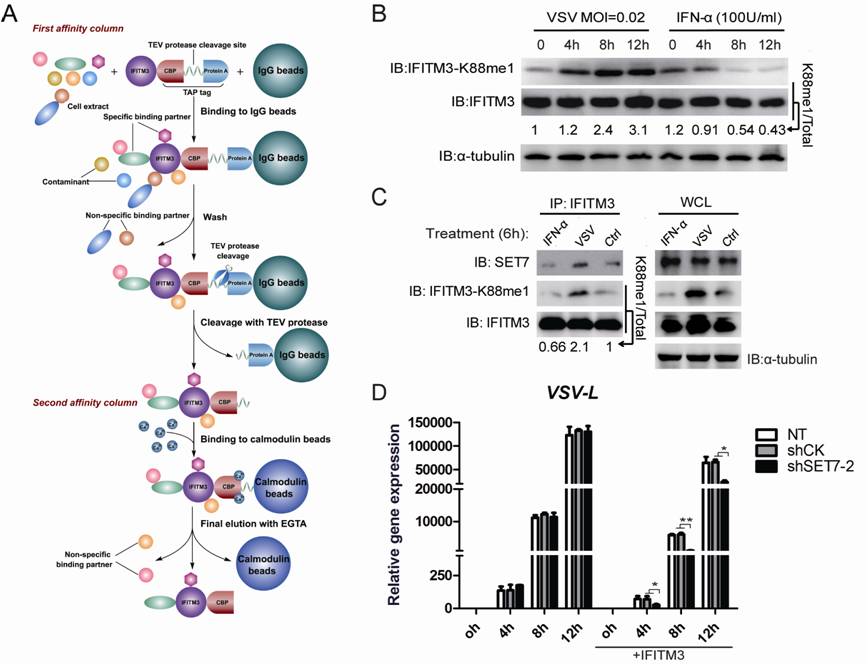
SET7-mediated lysine monomethylation negatively regulates IFITM3’s anti-viral function. (A) The strategy of tandem affinity purification. (B) Monomethylation of IFITM3 at K88 is induced by VSV infection but reduced upon IFN-α treatment. (C) Endogenous interaction between IFITM3 and SET7 is upregulated by VSV infection but downregulated by IFN-α treatment. (D) Knockdown of SET7 affects VSV infection only in the presence of IFITM3 expression.

